 |
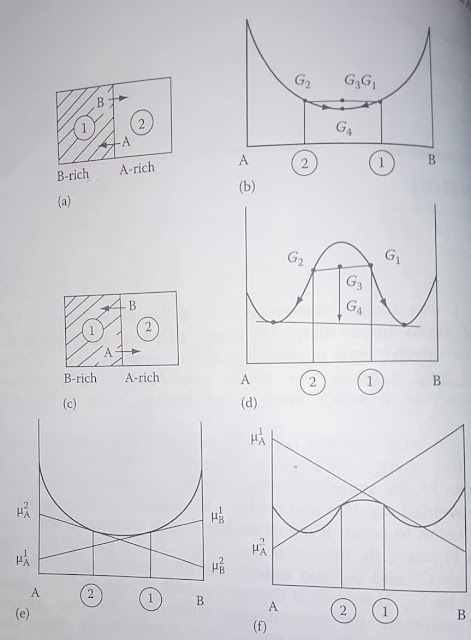
a),b),c),d) Shows diffusion due to concentration gradient both downhill and uphill respectively
e),f) Shows images of diffusion due to difference in chemical potentials |
Driving force for diffusion:
Theory:
There can be a little bit of confusion in your mind related to the driving force of diffusion, believe me, most of the students think that the driving force for diffusion is the concentration gradient.
But unfortunately, you will be surprised to know that those who think that the driving force for diffusion is the concentration gradient are wrong.
The driving force for diffusion is the difference in chemical potentials.
Explanation:
Diffusion basically occurs to reduce the Gibbs free energy of the system.
If you observe the diagrams a) and b) carefully you will note that the diffusion of A atoms is taking place from the region of higher concentration to the region of lower concentration and the b) graph also shows the reduction of Gibbs free energy. Similar for B atoms.
Whereas if you observe the diagrams c) and d) carefully you will note that the diffusion of A atoms is taking place from the region of lower concentration of A atoms to the region of higher concentration of A atoms and still the Gibbs free energy is decreasing as shown by d) graph. Similar for B atoms.
This completely disturbs the belief that diffusion takes place from the region of higher concentration to the region of lower concentration. This occurs due to the miscibility gap that is present in certain alloy systems which leads to negative curvature of free energy curves at lower temperatures.
Thus it shows that we cannot completely rely on the concentration gradient as driving force.
Whereas you can observe the graphs e) and f), in both cases, the chemical potential gradient helps in diffusing atoms from the region of higher chemical potential to a region of lower chemical potential.
And thus chemical potential is more reliable to be used as driving force.
CONCLUSION:
Driving force for diffusion is the difference in chemical potential and not the difference in the concentration gradient, the reason is that the concentration gradient is true only on certain circumstances whereas the difference in chemical potential is applicable in all circumstances and thus strictly speaking it is better to express driving force in terms of difference in chemical potentials.
What do you want to know more about diffusion?
Please free to ask below in the comments!!!


Comments
Post a Comment